The Basics on Vapor-Liquid Operations
Have you ever wonder how useful is Distillation? Or what about Gas Absorption? Or what do you think about Wetted Wall Columns? You may be you think those are very simple processes and that due to their simplicity, we don’t use them in the industry… Well, this is not true at all!
The so called Vapor-Liquid and Gas-Liquid Unit Operations (the ones we were talking about earlier, but many more!) are used EXTENSIVELY in the Industry. I could start listing all the types of distillations there are, or I could name some Absorption Processes such as Amine Tratment for Sweetening Natural Gas, Scrubbing CO2 from exhaust gases or so on…
The applications are infinite!
The Basics on Vapor-Liquid Operations
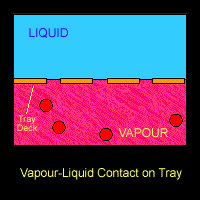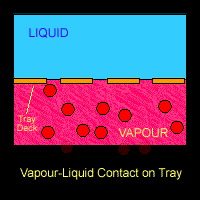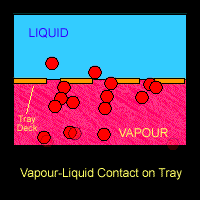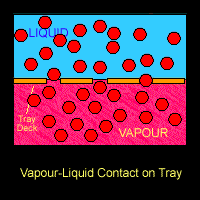
There are several physical phenomena that occurs when vapor-liquid are processed in a streamline. The most common ones are related to mass transfer. There is a relationship between partial pressure and vapor pressure which relates both phase: liquid an the vapor. When in equilibrium, they will interact between each other and distribute the components in both phases.
The main approach is to work with volatilities and K-values, yet my favorite approach to understand the basic understanding of flash systems is Raoult’s Law!
The Basics on Gas-Liquid Operations
As you can imagine, the Gas-Liquid interaction will have similar properties as the vapor-liquid interaction. Why? Well, there are two fluids, one incompressible and the other compressible, still, one will NOT condense in this case, whereas the Vapor will do.
There are several physical phenomena that occurs when flashing a streamline. The most common ones are related to mass transfer in gas solubility (into a liquid).

Note that here we will need another kind of Equilibrium, we no longer talk about the vapor-liquid equilibrium but the gas-liquid solubility line, or better yet, the Solubility Distribution Curve of Equilibrium.
The main approach is now to work with solubility of the SOLUTE of interest in both, the Gas phase and the Liquid Phase.
We typical work with dilute cases & non-dilute cases. One of the simplest form to relate such equilibrium curves is using Henry’s Law!
The Unit Operations related to these Phenomena:
The following is a list of common applications of processes in the industry.
It is important to ensure the difference betwee:
- Process
- Phenomena
- Unit Operation
For more info, Check out more on this video.
Distillation
This is one of the most common examples of industries that require SEPARATION of at least two materials.
Distillation exploits the differences in boiling point of the two substance in order to separate such materials. According to the degree of separation (purity required) you will see that the column will increase in height.
Some industrial applications:
- Benzene-Toluene Separation
- Ethylene Dichloride vs. Heavy Ends (image below)
- Distillation of Air (Nitrogen, Oxygen, Argon)
Petroleum Refining
Anyways, this post is about the INDUSTRIES that use Distillation such as Flash Drums and so on (Note that not all flashing systems are flash drums!)
By far, this is one of the TOP industries using distillation systems… why? because the amount of components that need to be separated, purified is huge.
The most common distillations:
- Crude Oil Distillation Units (CDU)
- Vacuum Distillation Units (VDU)
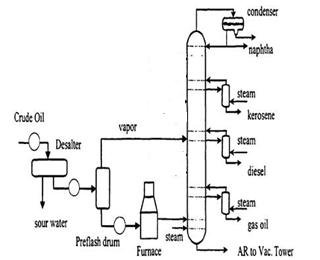
This is mostly due to the appearance of hydrogen gas, high volatility alkanes and other materials such as hydrogen sulfide formation.
Check out the Oil & Gas Bundle, or the Petroleum Refining Course.
Wetted Wall Columns
This is an applications which gets typically ignored in a Gas-Liquid application. Many academics and professionals will categorize this process as a Wetting/Cooling unit. Which of course might be the case, depending on the industry.
Wetting, because humidity applications are HUGE applications in the industry. Actually, you might encounter courses dedicated for only wet/humid/drying applications.
Anyways, Wetted Wall Columns require mass transfer, as they will interact with the AIR.
The solute or gas present will then interact in a Gas-Liqud Manner.
Acid Gas Treatment (Amine Treatment)
Now, talking about Natural Gas, LPG, Petrochemical & Petroleum Refining Industries, we can’t forget the Acid Gas Treatment. It is one of the most common examples of gas treatment for sulfur removal.
As you can see from the diagram below, there is a flash drum at low pressure which will favor low molecular weight gases to vaporize.
Methane and Ethane will be recovered later on, while the stream must be further treated.

Scrubbing of Gases
Scrubbing of Gases is by far one of the most important applications of gas absorption. As the name implies, we are “scrubbing” and cleaning the gases, typically from the exhaust of a combustion chamber.
This is very important, as it cleans the “toxic” air from pollutants. The most common applications or gases removed:
- CO2
- CO
- SO2
- NH3
- Particulate Matter
This is a relatively cheap form to “clean” a gas, even though it is expensive depending on the norms and regulations required.
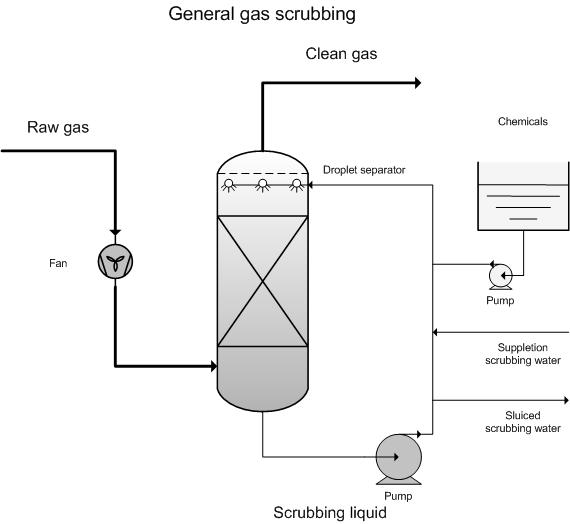
If you are interested on courses of Scrubbing of Gases, you should take a look to the Gas Absorption & Stripping in Chemical & Process Engineering Course!
These are common industrial applications.
It is of vital importance not only study for the sake of “studying” but to relate it in the engineering life!
Do you have any other application for Gas-Liquid or Vapor-Liquid? Im pretty sure I missed some of them (intentionally)
Post them on the comment section below!

